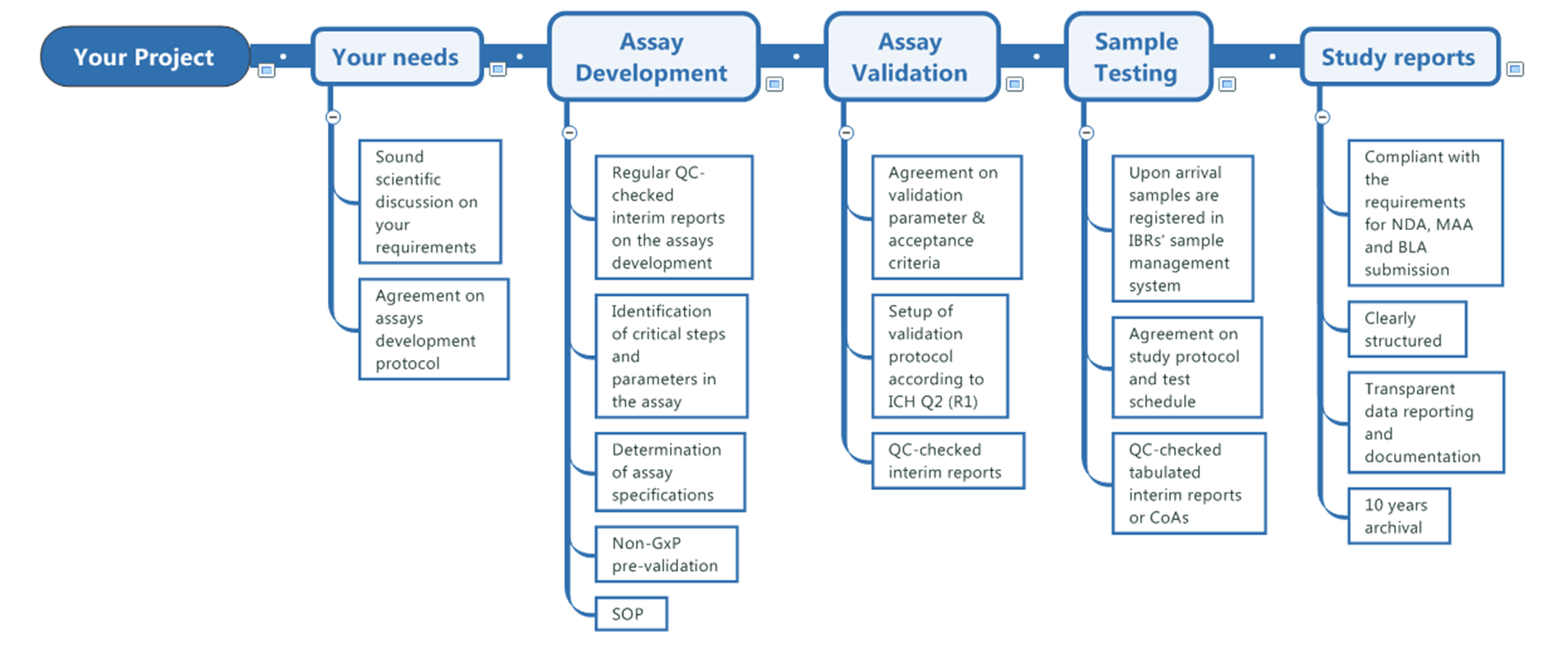
Study Management
IBR Inc. is committed to deliver high quality and reliable results. We set high value on scientific discussion with our customers, to provide them with studies tailored to their needs.
IBR Inc. is compliant with GMP and GCP requirements. With respect to assay development and validation, we follow the principles of ICH Q2 (R1), USP 1032, 1033 and 1034.
Submit a Question or Request
Simply fill out the form below and a member of our team will follow up with you shortly.